Superfund Research Program
New Passive Sampling Device for PFAS
View Research Brief as PDF(504KB)
Release Date: 11/03/2021
![]() subscribe/listen via iTunes, download(4.8MB), Transcript(84KB)
subscribe/listen via iTunes, download(4.8MB), Transcript(84KB)
Researchers from the NIEHS Superfund Research Program (SRP)-funded centers at the University of Rhode Island (URI) and Brown University developed a new type of passive sampling device for per- and polyfluoroalkyl substances (PFAS). Their new tool overcomes many limitations to traditional approaches, such as detecting short-chain PFAS and low concentrations of the chemicals in water.
PFAS chemicals, a large group of compounds found in aqueous film-forming foams, used for fire suppression, and in everyday consumer products, are made up of a chain of linked carbon and fluorine atoms. The length of the carbon-fluorine chain varies between PFAS chemicals, but because this carbon-fluorine bond is strong, PFAS do not easily break down in the environment. Until now, effectively monitoring PFAS at low, environmentally relevant concentrations was difficult.
Led by Jitka Becanova, Ph.D., a URI SRP Center trainee working with Rainer Lohmann, Ph.D., the collaborative project was made possible through a K.C. Donnelly Externship Award in 2018. Becanova used the award to work with Robert Hurt, Ph.D., at the Brown University SRP Center.
Designing and optimizing the sampling device
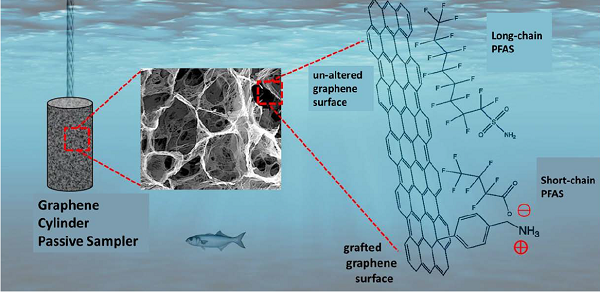
The sampling devices are miniature cylinders assembled from graphene oxide nanosheets, which stack to create internal pores. These cylinders collect PFAS from aquatic environments via adsorption, leveraging the high internal surface area of the atomically thin graphene. The PFAS are then concentrated and can be extracted and measured using traditional laboratory methods, such as mass spectrometry.
The team measured the ability of the cylinders to collect 23 different PFAS chemicals from water. Then they explored how to optimize the samplers to collect a broader range of PFAS chemicals. In particular, they sought to improve the functionality for sampling short-chain PFAS, which tend to have negative chemical charges and, therefore, are repelled by the similarly negatively charged graphene oxide nanosheets.
Using a novel but simple grafting method based on diazonium chemistry, they were able to introduce a positive surface charge to the graphene cylinders, thereby increasing their affinity to adsorb short-chain PFAS. The team reported ten-fold increased sorption of short- and middle-chain PFAS using this modification.
Testing in the field
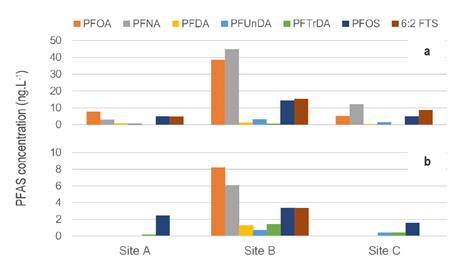
To evaluate the performance of the passive samplers under realistic conditions, the team deployed them in the Delaware River in New Jersey. This area is known to have high levels of several PFAS chemicals.
They measured PFAS levels in water samples collected from three sites using water grabs and compared them to measurements using the graphene cylinder method. Both approaches revealed the same composition of PFAS at these sites. The concentrations of PFAS derived from the passive sampler were four-fold lower than the data obtained from the water grabs.
According to the team, these findings demonstrate that their passive samplers successfully measure PFAS in the field and may prove promising as a screening tool for PFAS.
For More Information Contact:
Rainer Lohmann
University of Rhode Island
132 Horn Laboratory
Bay Campus
Narragansett, Rhode Island 02882-1197
Phone: 401-874-6612
Email: rlohmann@uri.edu
Robert H. Hurt
Brown University
School of Engineering
Barus & Holley Building
Providence, Rhode Island 02912
Phone: 401-863-2685
Email: robert_hurt@brown.edu
To learn more about this research, please refer to the following sources:
- Becanova J, Saleeba Z, Stone A, Robuck AR, Hurt RH, Lohmann R. 2021. A graphene-based hydrogel monolith with tailored surface chemistry for PFAS passive sampling. Environ Sci Nano 14:doi:10.1039/D1EN00517K
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


