Superfund Research Program
Arsenic and Manganese Mobility: Land Use, Redox Shifts, and Environmental Sensors
Project Leader: Charles F. Harvey (Massachusetts Institute of Technology)
Grant Number: P42ES016454
Funding Period: 2010-2015
![]()
Project-Specific Links
- Project Summary
Final Progress Reports
Year: 2013

Figure 1: Trainee Mason Stahl and students at the Bangladesh University of Engineering and Technology measure the pump rate of an irrigation well, which helps control the direction of groundwater flow in the area close to the study pond. (Photo courtesy of Harvard)
As part of the project's NIEHS-supported research effort, Dr. Harvey and his research team built a first of its kind experimental field station to track solute plumes emanating from a lake into the underlying aquifer. As they described in last years report, they constructed a 1200 m2 experimental pond (see Figure 2) with 150 wells and piezometers in the underlying sediments and aquifer. This year they have completed their biogeochemical characterization of the pond, the underlying sediments and the deeper aquifer that ultimately receives recharge from the pond.

Figure 2: Pond with bamboo platform in center from which samples from 8 wells and 12 shallow pore water samplers are taken. (Photo courtesy of Harvard)
Despite widespread agreement among researchers that organic carbon triggers arsenic and manganese mobilization, the research community remains divided over the source of organic carbon. Carbon dating offers a powerful and straightforward means to differentiate young organic carbon that has been drawn down from the surface from old detrital carbon deposited with the aquifer. In the samples taken from beneath the researcher's pond, they found that dissolved inorganic carbon is younger than dissolved organic carbon (see Figure 3).
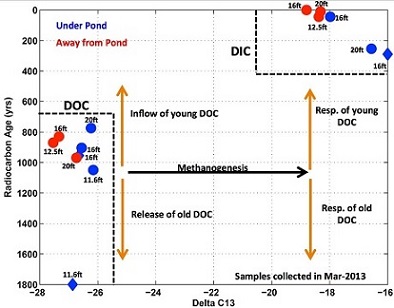
Figure 3: Graph showing that dissolved inorganic carbon is more recent than dissolved organic carbon in samples taken from beneath the pond. (Photo courtesy of Harvard)
Because inorganic carbon is produced by oxidation of organic carbon, this result suggests that microbes are preferentially selecting younger carbon from the pond to metabolize, and hence the processes that mobilize arsenic and manganese are driven by inflow of organic carbon from the overlying pond. The researchers also found a seasonal pattern in biogeochemical transformations in the sediment underlying the pond (see Figure 4). Organic carbon and ammonium concentrations increased in the summer during monsoon flooding in a ratio consistent with fermentation. Ultraviolet absorbance spectrums also indicated a shift in the characteristics of the dissolved organic carbon. Together these results suggest mobilization of labile organic carbon by fermentation during periods of flow stagnation.
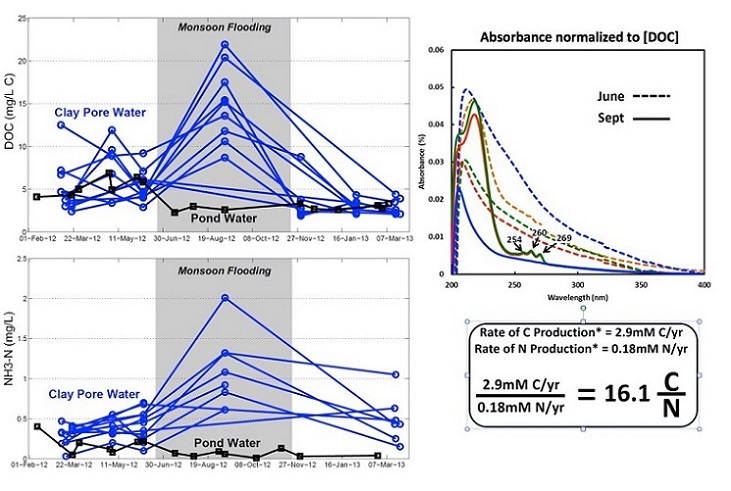
Figure 4: Graphing the seasonal variation in biogeochemical transformations in the underlying pond sediment. (Photo courtesy of Harvard)
Over the last eighteen months, the researchers have characterized the geochemical conditions in the sediment and aquifer underlying the lake, mapping the three-dimensional groundwater chemistry of a contaminated aquifer in unprecedented detail. This characterization enables them to see how invading water from the lake mixes and reacts with the in situ groundwater chemistry. To date they have analyzed over 1,600 water samples for up to 27 different parameters, for a total of over 18,000 water quality measurements. In the field they measure pH, DO, electrical conductivity, and temperature using standard probes. Alkalinity is measured by gran titration and ammonia is measured spectrophotometrically using the Nessler method within 48hrs of sample collection. Field separation of redox species are carried out by selective adsorption to resins. Samples for cation/trace metal analysis (filtered through 0.45 membrane and acidified w/ HNO3 to pH<2) are analyzed for Sr, Si, Ca, Mg, K, Na, Mn, Fe, P, S by ICP-OES and As by ICP-MS operating with a Dynamic Reaction Cell. Samples for DOC (filtered through 0.2µm PES membrane and acidified w/ HCl to pH<2) are analyzed for non-purgable DOC by a TOC-V CSH Total Organic Carbon Analyzer. Samples for oxygen and deuterium isotopes (filtered through 0.45um filter) are analyzed by isotope ratio infrared spectroscopy (IRIS) on a wavelength-scanned cavity ring-down spectrophotometer (WS-CRDS).
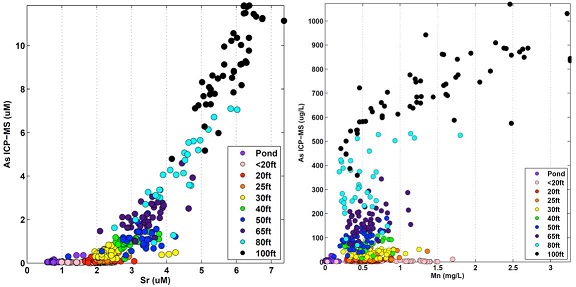
Figure 5: Graph depicting the strong relationship between arsenic and strontium (left) and showing a strong relationship between arsenic and manganese, when manganese exceeds 1.0 mg/l (right). (Photo courtesy of Harvard)
This extensive data set is providing new insight into the geochemical conditions that produce high concentrations of arsenic and manganese within aquifers. For example, the researchers find a very strong relation of dissolved arsenic to strontium, a relation that is consistent with arsenic mobilization by dissimilatory hydrous ferric oxide reduction. The researchers also find that there is little relation of arsenic concentrations to manganese concentrations in the range of 0.2 to 1.0 mg/L, but when manganese exceeds 1.0 mg/L arsenic concentrations are extremely high, above 500 ug/L (see Figure 5). This relation is consistent with the reductive dissolution of arsenic-bearing minerals including those of Mn (III/IV), and suggests that dangerous manganese level will occur in water that also has dangerous arsenic concentrations.


