Superfund Research Program
Epigenomic Actions of Environmental Arsenicals
Project Leader: Bernard W. Futscher
Grant Number: P42ES004940
Funding Period: 2010-2015
![]()
Project-Specific Links
- Project Summary
Final Progress Reports
Year: 2014
Arsenic exposure either through ingestion of inhalation, as might occur through dust exposures from the Iron King Mine and Humboldt Smelter Superfund site, can lead to the development of lung and/or bladder cancers. It is therefore important to understand the mechanisms by which this can occur. Futscher's laboratory's models of arsenical-mediated malignant transformation indicate epigenetic silencing of gene expression is induced by long term arsenical exposure, and further these expression changes are directly linked to the observed phenotypic shift. In order to separate "drivers" from "passenger" epigenetic changes, it is important to evaluate the functional significance of the genes identified. While experimental tools exist and have been used that selectively reduce expression of target genes (e.g., RNA interference), achieving complete ablation of gene expression has proven difficult. Thus, a significant technical hurdle to decisively examining the potential functional role of epigenetic silenced genes in the research team's arsenic-induced models of malignant transformation has been an inability to selectively and fully ablate the expression (and therefore function) of the candidate genes. To overcome this obstacle, they have developed and implemented genomic engineering in the laboratory using the CrispR/Cas9 system for editing the genome of human cells.
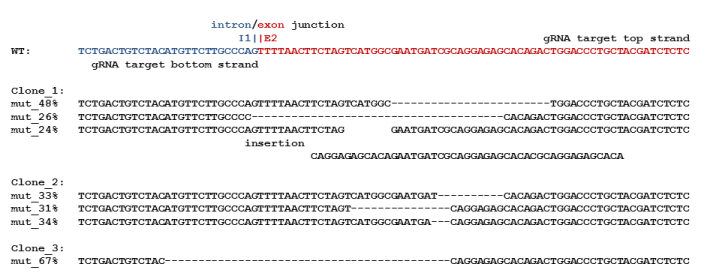
Figure 1. CrispR/Cas induced mutations in the MORT gene in BT549. A MORT-targeted dual nicking CRISPR/Cas9 vector was used to edit the MORT gene. The top portion of the figure shows the intron/exon boundary targeted, the gRNA sequences, and the DNA nicking sites. The bottom portion of the figure show the DNA sequences of the MORT region from 3 separate edited clones showing the types of deletions and insertions found in the edited clones. Clones 1 and 2 edited all three copies of MORT present in Bt549 while clone 3 edited two of the three copies of MORT. (Photo courtesy of University of Arizona)
In proof of principle studies the researchers have successfully targeted the lncRNA, ZNF667AS, a gene that is epigenetically silenced during arsenical-induced malignant transformation. A Futscher lab designed ZNF667AS-targeted dual nicking CRISPR/Cas9 vector, which includes a GFP marker, was transfected into BT549. Following transfection, GFP-positive cells were sorted into 96 well plates at clonal densities. After 2 weeks to allow for colony formation, clones were identified and expanded, DNA was isolated, and the ZNF667AS targeted region was deep sequenced in the on a Life Technologies PGM. Figure 1 shows the basic editing strategy and the genetic edits found at the ZNF667AS-targeted region in three of the clones BT549 (researchers isolated many more with ZNF667AS-targeted edits). These results demonstrate the researchers' ability to edit the genome and provide them a new opportunity to examine the functional significance of the candidate genes that have been epigenetically silenced by arsenic.


