Superfund Research Program
New Technique Yields Promising Results for Uranium Removal in the Field
View Research Brief as PDF(363KB)
Release Date: 02/02/2022
![]() subscribe/listen via iTunes, download(6.0MB), Transcript(88KB)
subscribe/listen via iTunes, download(6.0MB), Transcript(88KB)
A technology developed by NIEHS-funded Superfund Research Program (SRP) researchers may remove uranium and other heavy metals from groundwater near abandoned mines. Small business GlycoSurf, LLC worked with partners at the University of Arizona SRP Center to determine the best environmental conditions for effectively removing uranium from contaminated water.
The team leveraged a common process, called ion flotation, in which a surfactant attracts metal ions that are carried to the surface of the solution by air bubbles. They are then concentrated into a small volume of foam that can be disposed of or regenerated into another product. Their proprietary green rhamnolipid surfactants can selectively bind to uranium during ion flotation, but those surfactants had not been tested previously under realistic environmental conditions. GlycoSurf’s specialized green surfactants are produced synthetically and have similar properties to rhamnolipids made from biological organisms. In both cases, the materials are made using sustainable production methods, can biodegrade, can be recycled, and have low toxicity.
Testing in the Field
In the southwest U.S., thousands of abandoned uranium mining sites contaminate nearby drinking water sources. On the Navajo Nation alone, there are over 500 abandoned uranium mining sites and 12.8% of tested water sources exceed national drinking water standards.
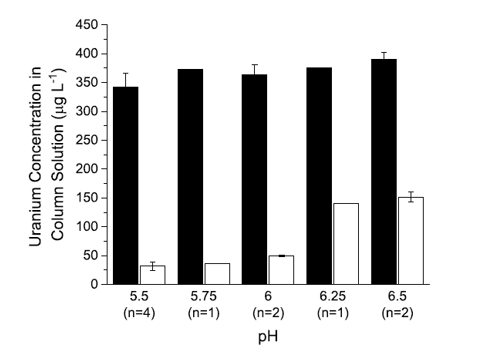
Collaborators tested both biologically derived rhamnolipids, and GlycoSurf’s bio-inspired rhamnolipids using groundwater samples collected from the Monument Valley site, a former uranium processing mill located on the Navajo Nation in northeastern Arizona. At the water’s normal pH of 8, ion flotation did not remove any uranium from solution.
Researchers conducted a second set of experiments lowering the pH of the system and observed that at pH 6.5 their technology removed 64.4% of uranium in the system. However, this concentration was still above the maximum contaminant level of 30 micrograms per liter set by the U.S. Environmental Protection Agency. By further lowering the pH to 5.5, they removed 92.6% of uranium in the system, resulting in levels near or below the maximum contaminant level.
Given that different forms of uranium are prevalent at different pH levels in the system, the uranium at higher pH are likely incompatible and not able to bind to rhamnolipid, say the team.
Optimizing the Experimental Design
The research team also aimed to evaluate the performance of three synthetic rhamnolipids developed by GlycoSurf compared to biologically derived rhamnolipids. Biologically derived rhamnolipids typically contain fatty acids with carbon chain lengths between 4 and 12, so the team tested synthetic rhamnolipids with carbon chain lengths of 10, 12, or 14. GlycoSurf’s synthetic rhamnolipids are made from sugar-based renewable resources, can be easily synthesized, and are highly reproducible.
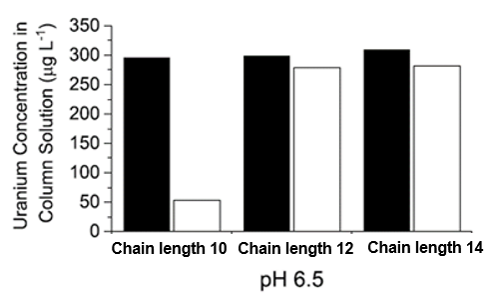
The team reported that the shorter chain synthetic rhamnolipid was equally as effective as the biologically derived rhamnolipid in removing uranium from the system at pH 6.5 and below. On the other hand, the two longer chain rhamnolipids were not suitable for ion flotation in this system at any pH.
Although their technology failed to remove uranium at the natural pH level, it was successful when conditions were appropriately adjusted. According to the authors, synthetic rhamnolipids can effectively remove uranium via ion flotation for acidic solutions and will be most successful when water chemistry and other conditions at specific sites are considered.
For More Information Contact:
Chett Boxley
GlycoSurf, LLC
1901 Prospector Ave
Suite #24
Park City, Utah 84060-7207
Phone: 435-901-1839
Email: boxley@glycosurf.com
Raina M Maier
University of Arizona
Department of Environmental Science
Saguaro Hall 322
Tucson, Arizona 85721-0038
Phone: 520-621-7231
Email: rmaier@ag.arizona.edu
To learn more about this research, please refer to the following sources:
- Hogan DE, Stolley RM, Boxley C, Amistadi M, Maier RM. 2022. Removal of uranium from contaminated groundwater using monorhamnolipids and ion flotation. J Environ Manage 301:doi:10.1016/j.jenvman.2021.113835 PMID:34600421 PMCID:PMC8579952
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


