Superfund Research Program
Emerging PFAS Can Cause Changes in Gene Expression and Lipid Accumulation in Human Liver Cells
View Research Brief as PDF(708KB)
Release Date: 07/06/2022
![]() subscribe/listen via iTunes, download(6.5MB), Transcript(89KB)
subscribe/listen via iTunes, download(6.5MB), Transcript(89KB)
New types of per- and polyfluoroalkyl substances (PFAS) can induce significant increases in gene expression and lipid accumulation in human liver cells at lower concentrations compared to PFAS no longer in use, according to researchers funded by the NIEHS Superfund Research Program (SRP). Led by Angela Slitt, Ph.D., of the University of Rhode Island SRP Center, the team also found that certain structural properties of PFAS, such as molecular weight, contribute to lipid buildup and gene activity.
Various consumer and industrial products contain PFAS, which confer heat, stain, oil, and water resistance. Many PFAS break down slowly and can build up in people, animals, and the environment over time. Concerns over PFAS exposure has led to a phase-out of certain legacy chemicals, including perfluorooctanesulfonic acid and perfluorooctanoic acid — commonly known as PFOS and PFOA — and PFAS with long carbon chains. However, less is known about the potential toxicity of new, or emerging, shorter-chain PFAS and other PFAS replacement chemicals.
Some rodent and cell studies suggest that PFAS might be associated with fatty liver disease, which stems from a buildup of fat in the liver. Although some human studies have found an association between exposure to PFAS and signs of liver injury, their role in human liver diseases is still unclear. Slitt’s team wanted to compare how exposure to emerging and older PFAS affected lipid processing and buildup in human liver cells.
Looking for Lipids
Slitt and colleagues first obtained frozen human liver cells, thawed them, and plated them. Then they exposed the plates to three different concentrations of either legacy, long-chain, or emerging PFAS — totaling 19 compounds — or a control.
After incubation, the researchers extracted the cellular contents and analyzed them for activation of 35 genes with roles in lipid metabolism and related pathways. Specifically, they measured quantities of mRNA — a molecular messenger that conveys genetic instructions for protein synthesis — as a proxy for gene expression.
To another set of PFAS-exposed cells, the team added a red fluorescent dye designed to stain lipids as a way to visualize whether exposure led to fat accumulation. They also treated a set of unexposed cells with a lipid-based solution to serve as a control.
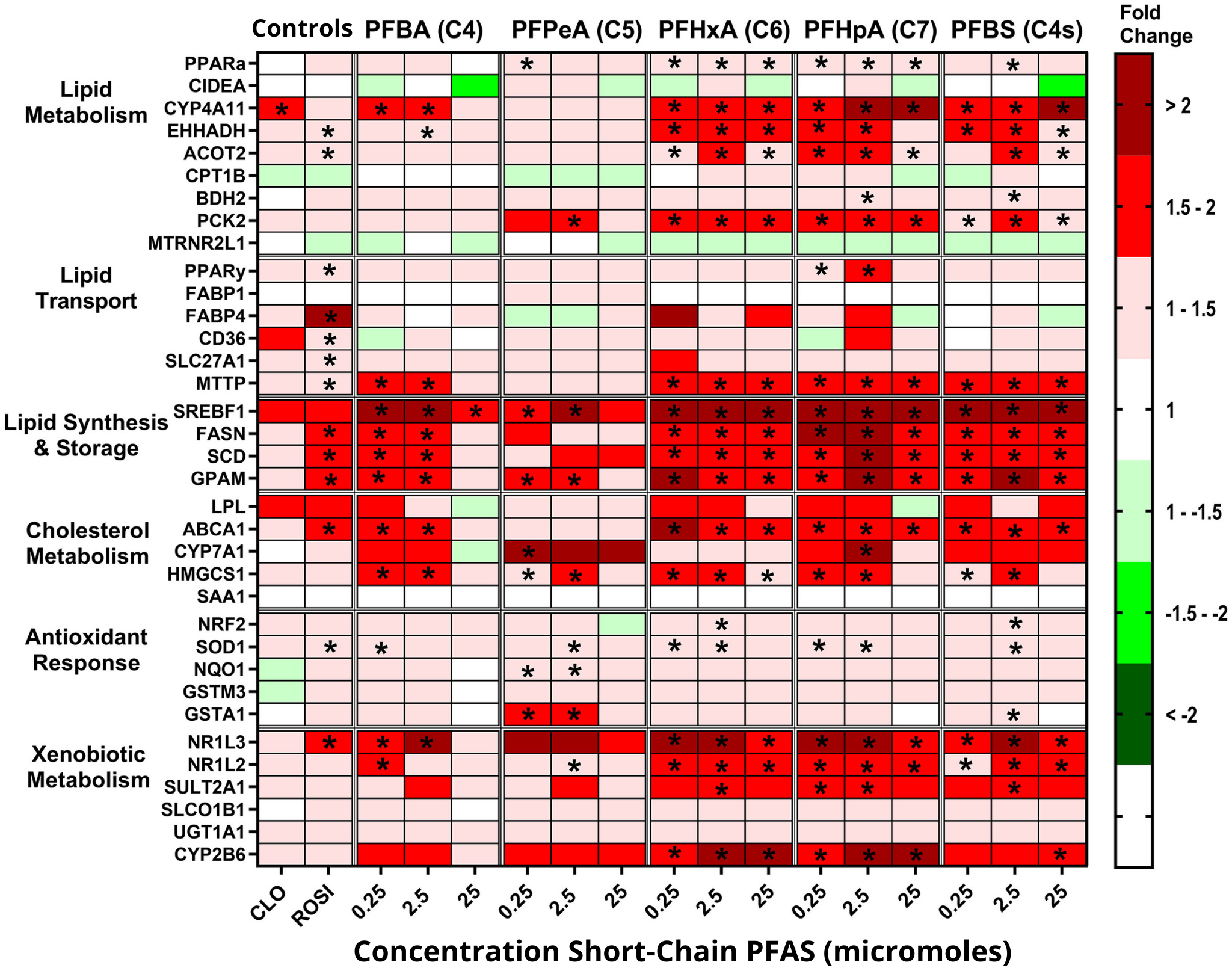
Overall, the researchers found that almost all PFAS activated at least some genes. Further, they observed potent gene activity for at least eight of the PFAS.
Legacy PFAS, for example, highly activated genes related to drug metabolism. Meanwhile, several emerging PFAS induced strong activation of multiple genes in pathways related to lipid synthesis and storage.
All told, short-chain PFAS increased gene activity for at least seven of the 35 genes tested at all concentrations, except for two at the highest concentration. By comparison, legacy PFAS were most influential at the highest concentration.
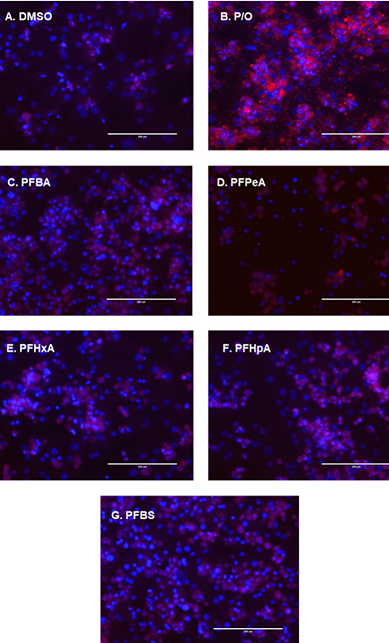
In addition, seven emerging PFAS induced significant liver lipid accumulation. On the other hand, the researchers found no lipid buildup in cells treated with legacy PFAS.
The chemicals that increased cellular lipid content correlated closely with PFAS that highly induced gene expression related to lipid synthesis and storage pathways, according to the authors.
Structural Influence
Slitt’s team also investigated the influence of PFAS structure and chemical properties on lipid accumulation and gene expression. Through computer modeling, they selected dozens of molecular traits to examine. They found that PFAS molecular weight was an important factor contributing to lipid accumulation and key changes in gene expression — specifically, the lower the molecular weight, the greater the likelihood of lipid buildup and gene activity.
“Overall, our study indicates that replacement PFAS and PFAS still in use can induce cellular changes in gene expression and lipid accumulation in human hepatocytes and suggest that further effects should be examined in humans,” the authors wrote.
For More Information Contact:
Angela L. Slitt
University of Rhode Island
Avedisian Hall
7 Greenhouse Road
Kingston, Rhode Island 02881
Phone: 401-874-5020
Email: aslitt@uri.edu
To learn more about this research, please refer to the following sources:
- Marques E, Pfohl M, Wei W, Tarantola G, Ford L, Amaeze O, Alesio J, Ryu S, Jia X, Zhu H, Bothun GD, Slitt AL. 2022. Replacement per- and polyfluoroalkyl substances (PFAS) are potent modulators of lipogenic and drug metabolizing gene expression signatures in primary human hepatocytes. Toxicol Appl Pharmacol 442:115991. doi:10.1016/j.taap.2022.115991 PMID:35337807 PMCID:PMC9036616
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


