Superfund Research Program
Improving How Microbes Break Down PFAS
View Research Brief as PDF(1.6MB)
Release Date: 08/03/2022
![]() subscribe/listen via iTunes, download(5.0MB), Transcript(114KB)
subscribe/listen via iTunes, download(5.0MB), Transcript(114KB)
NIEHS Superfund Research Program (SRP) grantees demonstrated a method to break down per- and polyfluoroalkyl substances (PFAS) into smaller, non-toxic molecules. Led by Yujie Men, Ph.D., of the University of California, Riverside, the team also showed that some types of PFAS can be more easily degraded than others.
PFAS are a group of more than 9,000 manmade chemicals used in a variety of industrial and consumer products. Known as forever chemicals, PFAS persist in the environment because they are made up of strong carbon-fluorine chemical bonds, which are difficult to break.
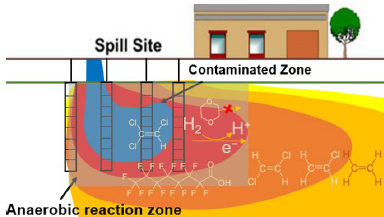
Although some microbes can break down contaminants like PFAS in a process called bioremediation, the diversity of PFAS structures adds complexity to this type of chemical degradation.
Breaking the Carbon-Fluorine Bond
The team explored how microorganisms, such as bacteria, can break the strong carbon-fluorine bonds that hold PFAS together —a process called defluorination. The goal is to transform them into non-toxic products in environments that are anaerobic, or without oxygen, such as groundwater. Their study included 16 different fluorinated carboxylic acids (FCAs), a relatively new group of PFAS.
They observed that unsaturated FCAs, which have at least one double bond between their carbon atoms with a fluorine atom attached to the double carbon bond, were more vulnerable to defluorination under anaerobic conditions. In contrast, saturated FCAs with similar structures were not degraded by the anaerobic microbial community. Saturated PFAS have no double bonds at all.
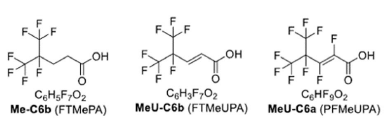
Further, unsaturated compounds with fluorine atoms linked to every carbon in the chain, called perfluorinated FCAs, had higher defluorination degrees than the polyfluorinated ones. Polyfluorinated FCAs have at least one carbon atom in the chain that is not bound to fluorine.
They also observed that the location of the fluorine atoms is important. For example, MeU-C6b, which has no fluorine atoms linked to the carbons with double bonds, had low defluorination degree compared to the other unsaturated chemicals.
These results have implications for designing novel PFAS alternatives that can be easily degraded, the authors noted.
Using Oxygen to Improve Remediation
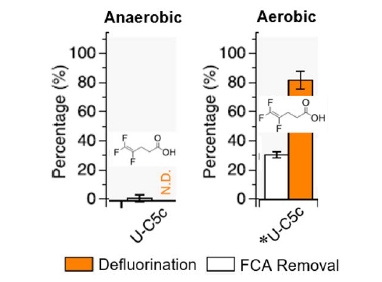
The researchers also tested an aerobic remediation approach in which microorganisms in activated sludge, obtained from a local wastewater treatment plant, use oxygen to degrade FCAs.
They observed that aerobic conditions enhanced the defluorination of the polyfluorinated FCAs, which showed low removal and defluorination under anaerobic conditions. For example, U-C5c, which did not degrade in anaerobic remediation, exhibited an 80% defluorination rate under aerobic conditions. Activated sludge had no effect on perfluorinated FCAs.
According to the authors, future research should include both anaerobic and aerobic bioremediation strategies in attempts to degrade a broader range of PFAS.
For More Information Contact:
Yujie Men
University of California-Riverside
900 University Ave
Riverside, California 92521
Phone: 951-827-2820
Email: ymen@engr.ucr.edu
To learn more about this research, please refer to the following sources:
- Yu Y, Che S, Ren C, Jin B, Tian Z, Liu J, Men Y. 2022. Microbial defluorination of unsaturated per- and polyfluorinated carboxylic acids under anaerobic and aerobic conditions: A structure specificity study. Environ Sci Technol 56(8):4894-4904. doi:10.1021/acs.est.1c05509 PMID:35373561
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


