Superfund Research Program
Sampling Device Harnesses Powerful Molecular Interactions, Overcomes Barriers in Detecting Volatile Contaminants
View Research Brief as PDF(688KB)
Release Date: 12/07/2022
![]() subscribe/listen via iTunes, download(5.8MB), Transcript(118KB)
subscribe/listen via iTunes, download(5.8MB), Transcript(118KB)
A NIEHS Superfund Research Program (SRP)-funded study showed how unique microsensors that harness powerful molecular interactions can selectively detect trace amounts of aromatic volatile organic compounds (VOCs) in the environment.
Aromatic VOCs are a subgroup of ring-shaped compounds found in the atmosphere of urban and semi-urban areas. This group includes benzene, toluene, ethylbenzene, and xylenes, sometimes referred to as BTEX, which are often found at Superfund sites. They have been linked to adverse health effects like cancer, even at low concentrations. Measuring and quantifying the pollutants at low concentrations is challenging because existing tools lack required sensitivity, are not selective for specific VOC compounds, and do not perform well with mixtures of VOCs.
To overcome these limitations, the research team, led by Michael Nantz, Ph.D., and Xiao-An Fu, Ph.D., of the University of Louisville SRP Center, took advantage of strong interactions between molecules with positive and negative electrical charges to develop a highly sensitive VOC sensor.
Fabricating sensitive and selective sensors
First, the team fabricated the sensors by creating functionalized gold nanoparticle cores surrounded by a layer of ligands, called thiols, that attach tightly to positively charged metal atoms. Then, the sensing material was integrated into an electrical circuit to create a specialized chemical sensor that detects and responds to interactions between the positively charged metals and the electron-rich rings of specific BTEX compounds.
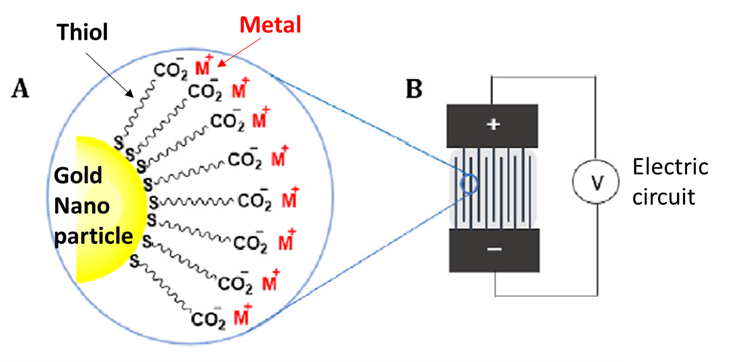
Next, they evaluated which metals – lithium, sodium, or potassium – performed best in the sensor, in terms of sensitivity and selectivity, for aromatic VOCs.
Then, they measured the electrical response, called resistance, to individual VOCs — benzene, toluene, ethylbenzene, xylene, nitrobenzene, cyclohexane, ethanol, and acetone — across a range of concentrations from 5 parts per million to 100 parts per billion.
Finally, they sought to clarify the specific molecular interactions underlying the sensor’s performance and whether environmental conditions, such as humidity, altered the results.
Powerful molecular interactions capture VOCs at low levels
The team reported that the sensors showed rapid high sensitivity and selectivity toward electron-rich aromatic VOCs, in particular benzene, toluene, ethylbenzene, and xylene. They explained that the characteristics of these compounds point to a particular type of strong molecular interaction driving sensor performance. Cation-pi interactions are strong attractive forces between a positively charged cation, such as the metals, and an electron-rich, negatively charged system, such as the rings of the BTEX compounds.
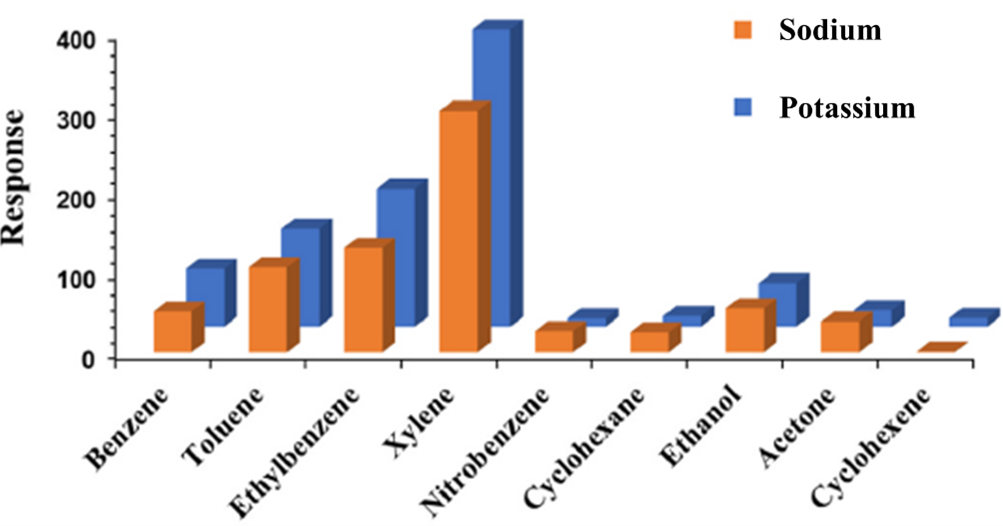
Among the different metals tested, the researchers reported the highest responses for the sensors with sodium or potassium. The team also calculated the sensors' limit of detection and found that sensors using sodium and potassium can detect BTEX compounds at concentrations as low as 0.1 to 3 ppb.
While humidity lessened the effectiveness of the sensors, the authors offered potential alternatives, such as using a sorbent tube to remove water, that could be explored further in future studies to expand their applicability to diverse environmental conditions. They also tested the long-term stability of the sensors, reporting that new sensors could be prepared by removing the previous sensing film and adding a new layer of the sensing material.
According to the authors, the robust responses to electron-rich aromatic VOCs observed here suggest their nanoparticle-based sensors could enhance the ability to rapidly detect these compounds at low concentrations, which may better clarify what VOCs people are exposed to and their potential health risks.
For More Information Contact:
Michael H. Nantz
University of Louisville
2210 South Brook St.
Shumaker Research Bldg., Room 345
Louisville, Kentucky 502-852-8069
Phone: 502-852-8069
Email: mhnant01@louisville.edu
To learn more about this research, please refer to the following sources:
- Adhihetty P, Halder S, Jasinski J, Fu X, Nantz MH. 2023. Harnessing the cation-pi interactions of metallated gold monolayer-protected clusters to detect aromatic volatile organic compounds. Talanta 253:123915. doi:10.1016/j.talanta.2022.123915 PMID:36155323
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


