Superfund Research Program
Mimicking Molecules Made by Bacteria to Remove Metals From Water
View Research Brief as PDF(1.7MB)
Release Date: 04/05/2023
![]() subscribe/listen via iTunes, download(4.9MB), Transcript(887KB)
subscribe/listen via iTunes, download(4.9MB), Transcript(887KB)
NIEHS Superfund Research Program (SRP)-funded scientists developed a method to extract metals from water using synthetic molecules inspired by those produced by bacteria. The biodegradable molecules, called rhamnolipids, could one day be used to remove toxic metals or extract rare and valuable elements from aqueous mining and industrial waste.
Growing desire for battery-powered devices, like electric vehicles, will likely raise demand for rare earth elements and metals. Traditional mining for rare earth elements and other metals disturbs natural resources, requires high energy input, and contributes to greenhouse gas emissions. Obtaining some rare metals from aqueous waste – from oil and gas production, saltwater brines, and acid mine drainage – could potentially meet demand and reduce environmental impacts. However, selective, cost-effective approaches to extract metals from water are not currently available.
Exploring Optimal Rhamnolipid Design and Treatment Steps
Researchers led by Raina Maier, Ph.D., and David Hogan, Ph.D., of the University of Arizona SRP Center, studied seven different synthetic rhamnolipids – which can be customized for specific uses –to evaluate their ability to remove three metals from solutions by forming a solid complex.
The synthetic rhamnolipids were tailored to vary in their properties based on differences in tail length, from six to 18 carbons, and to have one or two tails. Longer tails and multiple tails impart greater hydrophobic, or water-repellant, properties.
The scientists tested the ability of the rhamnolipids to form a complex with each metal – lead, lanthanum, or magnesium – at different concentrations. Then they compared three treatments to remove the rhamnolipid-metal complexes from solution: mixing only or mixing followed by an active removal step of either filtering or centrifuging, which condenses solids through rapid spinning.
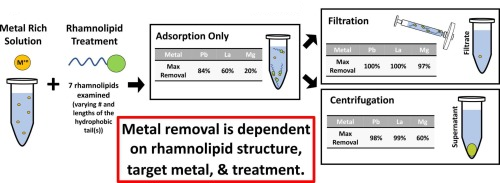
Depending on the rhamnolipid structure, the metal, the ratio of rhamnolipid to metal, and the removal step used, the team observed differences in how the molecules formed complexes with the metals and how efficiently metals were removed.
Digging Into Results
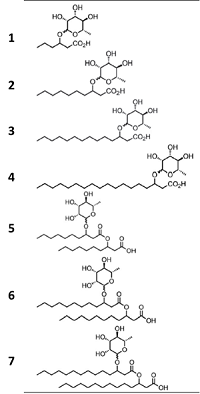
Overall, rhamnolipids with greater hydrophobicity were more effective at forming complexes with the metals, performing best with lead, followed by lanthanum, and magnesium. Adding the filter or centrifuge step generally improved performance with the same general trend by metal type.
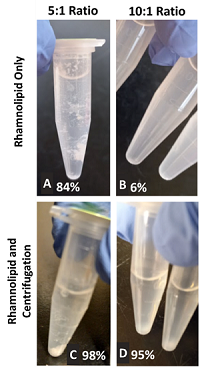
- Mixing Only: One of the seven rhamnolipids performed best with all three metals, removing 83.9% of lead, 59.4% lanthanum, and 19.5% of magnesium.
- Mixing and Filtering: Five rhamnolipids removed more than 97% of lead, three rhamnolipids removed more than 99% of lanthanum, and five removed more than 93% of magnesium.
- Mixing and Centrifuging: Three rhamnolipids removed more than 97% of lead and two removed more than 98% of lanthanum. Only one rhamnolipid removed up to 60% of magnesium with centrifugation.
According to the team, these results suggest that tailoring rhamnolipid structure and removal methods may enable more selective and environmentally-friendly extraction of either toxic metals or valuable rare earth elements compared to existing approaches. The efficiency of these techniques in more complex systems, such as metal mixtures or more realistic waste streams, should be evaluated in future studies, they added.
For More Information Contact:
Raina M Maier
University of Arizona
Department of Environmental Science
Saguaro Hall 322
Tucson, Arizona 85721-0038
Phone: 520-621-7231
Email: rmaier@ag.arizona.edu
To learn more about this research, please refer to the following sources:
- McCawley IA, Maier RM, Hogan DE. 2023. Comparison of synthetic rhamnolipids as chemical precipitants for Pb, La, and Mg. J Hazard Mater 447:130801. doi:10.1016/j.jhazmat.2023.130801 PMID:36689902 PMCID:PMC9986113
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


