Superfund Research Program
Modified Iron Particles Could Improve Bioremediation of PFAS
View Research Brief as PDF(4.6MB)
Release Date: 09/06/2023
![]() subscribe/listen via iTunes, download(6.0MB), Transcript(116KB)
subscribe/listen via iTunes, download(6.0MB), Transcript(116KB)
Iron particles coated in a nontoxic material may enhance PFAS degradation by a certain bacterium, according to researchers funded by the NIEHS Superfund Research Program. The study could inform bioremediation efforts that harness the microbe, known as Acidimicrobium Strain A6, for cleaning up contaminated soil, sediments, and aquifers.
Distinctive PFAS properties, such as high heat tolerance and oil resistance, stem from exceptionally stable bonds between carbon and fluorine atoms. Because PFAS resist breakdown, they can accumulate in exposed organisms and ecosystems, posing a risk to human and environmental health.
Some PFAS, such as perfluorooctanoic acid (PFOA) — implicated in immune and kidney problems, among others — are particularly recalcitrant to degradation. However, prior research by Princeton University’s Peter Jaffé, Ph.D., and colleagues found that Acidimicrobium Strain A6 (or A6 for short), can break down PFOA in contaminated wastewater.
This microorganism thrives in iron-rich, acidic environments, using inorganic material as an energy source. For the present study, Jaffé’s team sought to improve PFOA breakdown by stimulating A6 activity with iron.
Priming the Process
In nature, A6 generates energy by converting the nitrogen-based compound ammonium into nitrite. During the reaction, known as Feammox, electrons are released.
Most of those electrons are transferred to iron, such as a form called ferrihydrite. In PFOA’s presence, electrons can also latch on to fluorine atoms, effectively breaking their strong bonds with carbon.
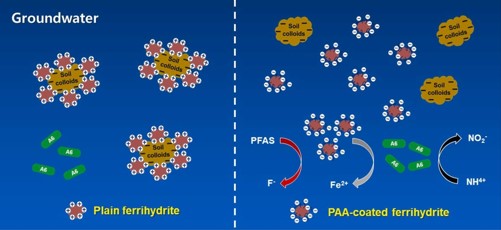
Adding ferrihydrite to wastewater can stoke bacterial breakdown of PFOA, according to laboratory studies by the team. But polluted field sites present a hurdle: Negatively charged sediment can trap the iron particles, preventing their distribution and hindering Feammox reactions from occurring.
In earlier work, the researchers found that ferrihydrite particles coated with polyacrylic acid — a biodegradable synthetic chemical — can move better through sediment samples. Based on that finding, they wondered: Could these coated iron particles promote A6 breakdown of PFOA?
To investigate, the team mixed ferrihydrite with polyacrylic acid of four different molecular weights, ranging from 2,100 to 450,000 grams per mole. The samples also contained an ammonium-based medium to encourage A6 growth. In a related experiment, they added PFOA to the mixtures.
After the samples incubated for several weeks under acidic conditions, the researchers analyzed their contents. Samples containing coated iron showed higher total bacteria populations compared to a control with bare ferrihydrite, suggesting that polyacrylic acid may stimulate bacterial growth, according to the authors. In addition, DNA sequencing indicated that A6 was the dominant group across samples.
The team also found significantly less ammonium in the mixtures with coated iron compared to the control. Using a powerful imaging technique, they observed that electrons were more easily transferred to coated iron than to bare iron. The findings suggested that the coated iron facilitated Feammox reactions.
Lowering PFOA Levels
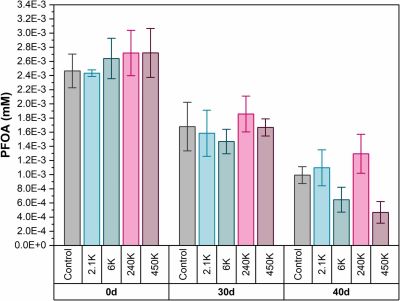
In two of the samples containing coated iron, the team found significant declines in PFOA concentrations, as well as significantly more free-floating fluoride — signs that PFOA was decomposing.
The team was curious whether declining PFOA levels meant that the chemical had entirely degraded or simply broken up into smaller PFAS molecules. They found only minor levels of PFAS intermediates appearing sporadically, suggesting that in most cases, PFOA was fully breaking down.
Together, the results demonstrated that polyacrylic-coated ferrihydrite boosted A6 growth and PFOA degradation, according to the authors. However, more research is needed to thoroughly explain the underlying mechanisms, they added. Further research should also explore how to optimize coated iron for use in more complex bioremediation settings, where different water chemistries and microbial communities may affect success.
For More Information Contact:
Peter Jaffe
Princeton University
Department of Civil and Environmental Engineering
Princeton, New Jersey 08544
Phone: 609-258-4653
Email: jaffe@princeton.edu
To learn more about this research, please refer to the following sources:
- Park J, Huang S, Koel BE, Jaffe P. 2023. Enhanced Feammox activity and perfluorooctanoic acid (PFOA) degradation by Acidimicrobium sp. Strain A6 using PAA-coated ferrihydrite as an electron acceptor. J Hazard Mater 459:132039. doi:10.1016/j.jhazmat.2023.132039 PMID:37480613
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


