Superfund Research Program
Environmentally Persistent Free Radicals, PAHs Interact to Increase Toxicity of Particulate Mixtures
Release Date: 12/13/2023
![]() subscribe/listen via iTunes, download(6.4MB), Transcript(102KB)
subscribe/listen via iTunes, download(6.4MB), Transcript(102KB)
Toxic air pollutants called environmentally persistent free radicals (EPFRs) may react with certain polycyclic aromatic hydrocarbons (PAHs) on the surface of airborne particles to form more toxic chemicals, according to researchers funded by the NIEHS Superfund Research Program (SRP). The study, led by Slawomir Lomnicki, Ph.D., of the Louisiana State University SRP Center, demonstrated that interactions between components of fine particulate matter mixtures may enhance their overall toxicity.
Complex Particulate Mixtures
Particulate matter refers to small particles suspended in the air. Inhaling particles smaller than 2.5 micrometers (PM2.5), like those that comprise smoke or smog, is especially hazardous and is associated with a higher risk of heart disease and asthma. Because PM2.5 is a complex mixture of many different components, it is unclear which constituents contribute most to the toxicity of PM2.5.
Two common constituents of PM2.5 mixtures are EPFRs and PAHs. EPFRs form during combustion and are linked to respiratory and cardiovascular problems. When they encounter liquids, such as body fluids, EPFRs form a reactive molecule that can alter other molecules through a process called oxidation. PAHs, which are primarily released from burning fossil fuels and plant matter, can be converted into oxy-PAHs by oxidation. Oxy-PAHs are highly toxic and are associated with DNA damage and various cancers.
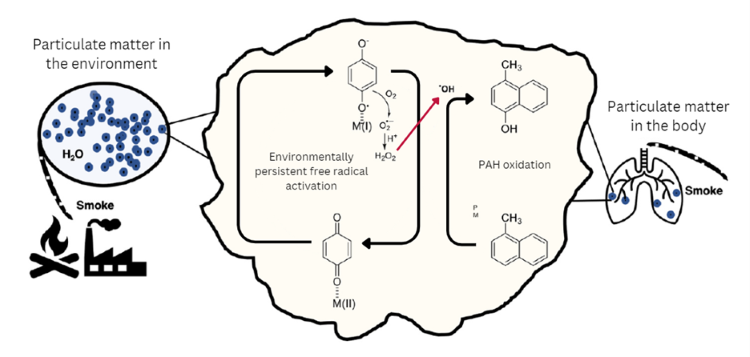
Testing EPFR-PAH Reactions
The researchers hypothesized that EPFRs may react with PAHs in PM2.5 mixtures to form oxy-PAHs. To test their hypothesis, they used particulates of copper-coated silica and covered the particle surfaces with EPFRs.
The team then added 1-methyl naphthalene, a type of PAH, to the EPFR mixture and to a control group of only copper-coated silica particulate matter. Next, the scientists suspended the two mixtures in water at room temperature (25°C) and human-body temperature (37°C). Both suspensions were shaken for 18 hours to allow any reactions to occur. Then, they measured the suspensions for the presence of oxy-PAHs.
Measuring Oxy-PAHs
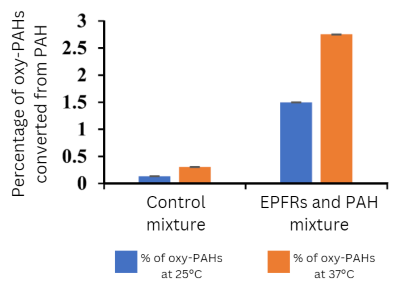
The researchers found oxy-PAHs in the suspensions of particulate mixtures containing EPFRs at both temperatures. By contrast, they detected almost no oxy-PAHs in the control suspensions. According to the scientists, the findings suggest that EPFRs oxidize PAHs when the two contaminants are present in particulate matter mixtures. Additionally, the EPFR suspension at 37°C contained higher levels of oxy-PAHs. This observation may indicate that the rate of PAH oxidation increases with temperature, the authors stated.
According to the team, the study demonstrates that reactions between EPFRs and PAHs may render particulate matter more toxic through the formation of oxy-PAHs. This factor may contribute to the overall toxicity of PM2.5 mixtures, they added.
For More Information Contact:
Slawo Lomnicki
Louisiana State University
To learn more about this research, please refer to the following sources:
- Ghimire A, Hasan F, Guan X, Potter PM, Guo C, Lomnicki S. 2023. Oxidation 1-methyl naphthalene based on the synergy of environmentally persistent free radicals (EPFRs) and PAHs in particulate matter (PM) surface. Chemosphere 341:140002. doi:10.1016/j.chemosphere.2023.140002 PMID:37648160 PMCID:PMC10548478
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


