Superfund Research Program
Using Earth Materials to Remove Metals Near Abandoned Mines
Release Date: 03/06/2024
![]() subscribe/listen via iTunes, download(5.6MB), Transcript(51KB)
subscribe/listen via iTunes, download(5.6MB), Transcript(51KB)
NIEHS Superfund Research Program (SRP)-funded researchers developed a new strategy that uses limestone and a naturally occurring mineral to clean up water contaminated with arsenic and uranium — two of the most frequently detected drinking water pollutants in Tribal communities.
More than 600,000 American Indians in the Southwestern U.S. are estimated to live within six miles of an abandoned mining site, which can contain mixtures of arsenic, uranium, and other toxic elements. These contaminants can pollute the local air, water, and soil.
To make remediation more cost efficient, investigators at the University of New Mexico SRP Center set out to develop a strategy that uses naturally occurring materials to remove arsenic and uranium from water. Because these metals often co-occur in the environment, the team aimed to develop an approach to remove both contaminants simultaneously.
Setting Up Remediation Experiments
Limestone is a type of rock commonly found near hard rock mines where uranium and arsenic co-occur. Limestone has properties that allow it to bind and trap contaminants, but its effectiveness can vary widely depending on its natural characteristics, such as particle size and mineral components.
To address limitations associated with limestone, the team incorporated hydroxyapatite, a mineral that has been observed to immobilize uranium and arsenic. Hydroxyapatite forms when the elements calcium and phosphorous interact in a high pH environment.
The team mixed solutions containing uranium and arsenic with either limestone or a combination of limestone, phosphate, and calcium. Then, they conducted their experiments at low, neutral, and high pH levels.
Revealing the Effects of Changing pH Levels
Results showed that at initially low pH levels, adding limestone to the solution increased pH to neutral levels and removed 90% of uranium and 100% of arsenic. However, limestone alone could not remove uranium and arsenic in solutions with initially neutral pH levels. At both low and neutral pH levels, incorporating calcium and phosphorus removed 90% of uranium but failed at removing arsenic from the solution.
The team then conducted a second batch of experiments with limestone and higher calcium and phosphate concentrations, which increased the pH of the solution and encouraged the formation of hydroxyapatite. The researchers found that this resulted in nearly complete arsenic and uranium removal. X-ray analysis confirmed that hydroxyapatite had formed and bound to most of the arsenic and uranium. However, the researchers observed that when pH increased beyond a level of 9.0, the efficiency of uranium removal decreased.
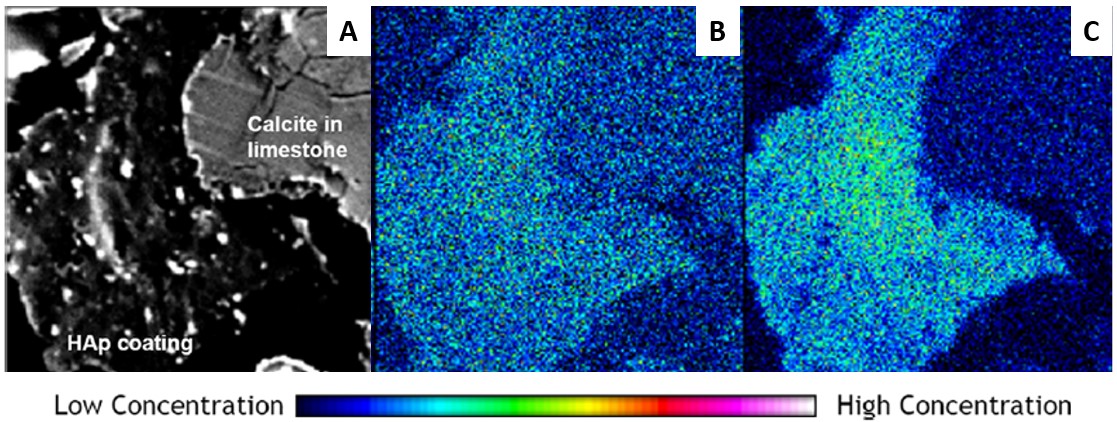
According to the authors, the findings suggest that adding calcium and phosphorous to limestone until a pH between 8.0 and 9.0 is achieved can form hydroxyapatite and enhance the removal of arsenic and uranium from water. The researchers believe a low-cost remediation strategy could be developed based on their experiment. It could help American Indian communities affected by contamination from abandoned mining sites.
For More Information Contact:
Jose Manuel Cerrato
University of New Mexico
1 University of New Mexico
Albuquerque, New Mexico 87131-0001
Phone: 505-272-1299
Email: jcerrato@unm.edu
To learn more about this research, please refer to the following sources:
- Meza I, Hua H, Gagnon K, Mulchandani A, Gonzalez-Estrella J, Burns PC, Ali AS, Spilde MN, Peterson EJ, Lichtner PC, Cerrato JM. 2023. Removal of aqueous uranyl and arsenate mixtures after reaction with limestone, PO4 3-, and Ca2. Environ Sci Technol 47(49):20881-20892. doi:10.1021/acs.est.3c03809 PMID:38019567 PMCID:PMC10739782
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


