Superfund Research Program
Electrochemical System Degrades PCE in Groundwater
View Research Brief as PDF(454KB)
Release Date: 04/01/2020
![]() subscribe/listen via iTunes, download(6.9MB), Transcript(85KB)
subscribe/listen via iTunes, download(6.9MB), Transcript(85KB)
An electrochemical system can effectively break down tetrachloroethylene (PCE) in groundwater, according to a new study from the NIEHS-funded Northeastern University Superfund Research Program (SRP) Center. After testing different design parameters to determine the best conditions for degrading PCE, the researchers achieved 86% removal of the contaminant from groundwater sources.
PCE is a solvent frequently used in dry cleaning solutions, adhesives, and metal degreasers. It can be released into the air, water, and soil and is commonly found in groundwater or soil at hazardous waste sites.
Groundwater can conduct electricity, so applying low direct electric currents can manipulate the chemistry of the water. This approach has been shown to transform certain hazardous contaminants, like chlorinated compounds, into non-toxic substances. The research team, led by Akram Alshawabkeh, Ph.D., previously showed efficient removal of the chemical trichloroethylene (TCE), a contaminant often found in groundwater and a byproduct of PCE degradation, using a simple two-electrode system.
The two electrodes, or electrical conductors, are referred to as the cathode and anode, which donate and accept electrons, respectively, from the surrounding groundwater. This movement of electrons has been shown to dechlorinate compounds such as TCE.
Optimizing Parameters
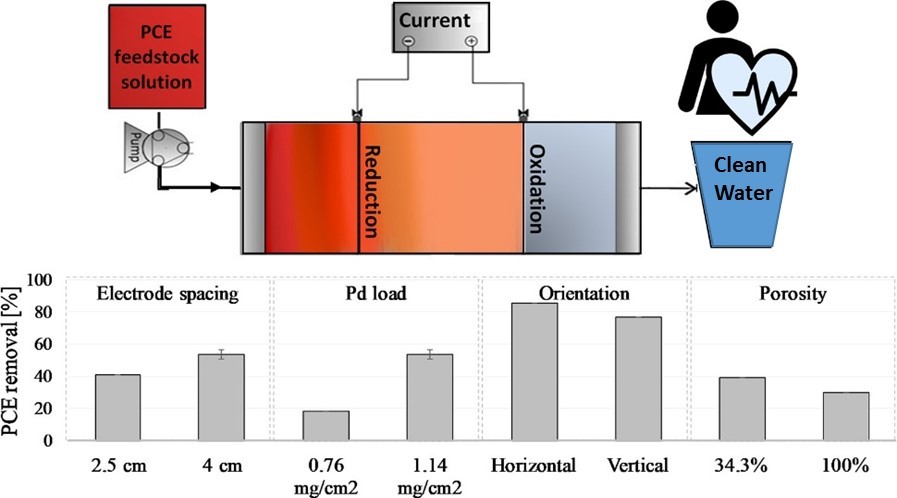 The researchers tested various parameters for removing PCE in the system. (Image modified from Hyldegaard et al. Sci Total Environ)
The researchers tested various parameters for removing PCE in the system. (Image modified from Hyldegaard et al. Sci Total Environ)In the new study, Alshawabkeh and his team used the electrode system to degrade the parent compound, PCE.
The research team studied electrochemical removal of PCE in a flow-through system design similar to what could be used in the field. Since groundwater generally flows horizontally, the researchers used a horizontally oriented system to facilitate scaling up the approach. They applied a constant current to the system and screened different parameters, such as spacing between electrodes, to see which factors improved PCE degradation.
The team also tested different electrode configurations to see what influenced electrochemical degradation of PCE in the flow-through system.
By adding a bipolar electrode between the cathode and the anode, they improved removal of PCE to about 54%. Adding palladium (Pd) and increasing the spacing between electrodes from 2.5 centimeters to 4 centimeters also improved removal.
The highest removal of 86% was reached in a liquid-filled, horizontally oriented system with a bipolar electrode between the cathode and anode and Pd loaded on the cathode.
Scaling Up for the Field
The researchers confirmed that this method did not lead to the formation of toxic intermediate chemicals. With some groundwater cleanup methods, PCE and TCE are not always fully converted to non-toxic ethene, and toxic intermediate chemicals such as vinyl chloride can be produced. The absence of chlorinated intermediates suggests that the system shows strong potential for field implementation. The researchers also found that lower contaminant concentrations were more difficult to treat and required applying higher electric currents for removal beyond 60%.
Screening different parameters is required to develop a system that works under varying conditions in the field, and important parameters that make the system more effective were identified. The authors note that further research is required to understand the mechanisms behind the degradation process and better address complex onsite variations, such as changes in groundwater flow.
For More Information Contact:
Akram N Alshawabkeh
Northeastern University
501 Stearns Center
360 Huntington Ave.
Boston, Massachusetts 02115-5000
Phone: 617-373-3994
Email: a.alshawabkeh@northeastern.edu
To learn more about this research, please refer to the following sources:
- Hyldegaard BH, Ottosen LM, Alshawabkeh AN. 2020. Transformation of tetrachloroethylene in a flow-through electrochemical reactor. Sci Total Environ 707:doi:10.1016/j.scitotenv.2019.135566 PMID:31767295 PMCID:PMC6980996
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


