Superfund Research Program
Fighting Fluorine with Fluorine: New Materials Remove PFAS from Groundwater
View Research Brief as PDF(581KB)
Release Date: 05/03/2023
![]() subscribe/listen via iTunes, download(7.0MB), Transcript(166KB)
subscribe/listen via iTunes, download(7.0MB), Transcript(166KB)
Researchers funded by the NIEHS Superfund Research Program (SRP) created a novel class of materials that can attract and remove per- and polyfluoroalkyl substances (PFAS) from water. According to the authors, the new technology — called Fluor Mop — can be regenerated, reused, and is potentially less expensive than current remediation strategies.
PFAS are manmade, heat-resistant compounds composed of carbon–fluorine bonds, which are among the strongest bonds in chemistry. Although these chemical properties make PFAS desirable for use in products like nonstick cookware and firefighting foams, they are also extremely persistent in the environment and can accumulate in organisms. Other NIEHS-funded research has linked PFAS exposure to health problems, such as altered metabolism and immune system deficits.
Current technologies used to remove contaminants from water, such as activated carbon and reverse osmosis, have low affinity for some PFAS and can discharge toxic waste. To address this challenge, the authors sought to design a new, PFAS-specific material.
Making the Materials
Led by Alexa May, Ph.D., of Weaver Labs, an SRP-funded small business, the team hypothesized that materials made of fluorine could take advantage of the attractive forces between fluorine bonds to adsorb and remove PFAS from water.
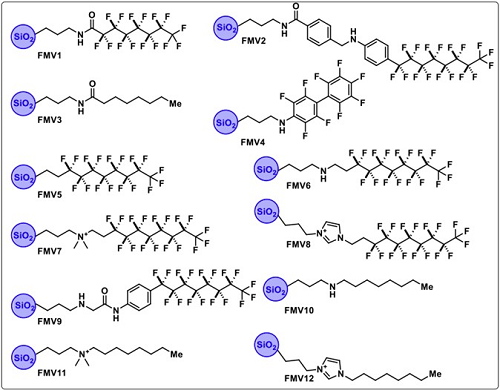
They created 12 Fluor Mop materials made of fluorine and silica, each denoted by a Fluor Mop Version (FMV) number. Fluorine–fluorine interactions are well described, and technologies that make use of these interactions have shown promise for PFAS adsorption, the authors said. Silica is commonly used in other sorbent technologies due to its porous structure and large surface area, so it was used to form the backbone of the new materials.
Each FMV was designed to have a unique surface chemistry and structural configuration, allowing each one to have an affinity for a different type of PFAS.
To test the materials’ PFAS removal efficiencies, the researchers collected groundwater samples from a site with known PFAS contamination. They also applied traditional water treatment technologies, including activated carbon and ion exchange (IX) resins, to compare their PFAS removal abilities. IX resins are long polymer molecules that can remove contaminants from water, replacing them with beneficial, desired ions.
A major objective of the study was to develop Fluor Mop materials that could be reused without diminished performance, so the team then analyzed how well the materials performed after multiple cycles of washing and reuse.
Combating the PFAS Problem
May and team tested how their materials performed for removing each of the eight PFAS species found in the groundwater samples. These included perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) — legacy PFAS with long carbon chains that were phased out of manufacturing in the early 2000s — as well as emerging shorter-chained PFAS, such as perfluorobutane sulfonate (PFBS) and perfluorohexanoic acid (PFHxA).
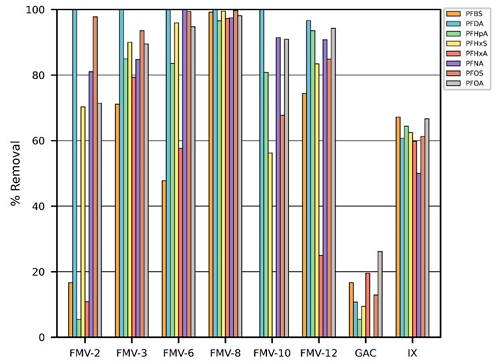
The best performing Fluor Mop material — FMV8 — removed nearly 97% of all PFAS tested, compared to less than 20% by granular activated carbon.
Materials FMV8 and FMV12 were structurally identical, except for the presence of fluorine in FMV8, and both materials performed similarly in their removal of PFOS and PFOA. However, the team noted that FMV8 showed a much greater ability to remove PFBS and PFHxA than FMV12, highlighting the benefit of a fluorinated component to target short-chained PFAS that other technologies have trouble removing.
In addition, the researchers noted that FMV8 could be reused for at least five cycles while maintaining its removal capability.
The SRP-funded small business plans to continue laboratory experiments to test the long-term stability of Fluor Mop materials, as well as their performance and operational costs at full-scale water treatment facilities.
For More Information Contact:
Alexa R May
Weaver Labs, LLC
Email: alexa.may@weaver-labs.com
To learn more about this research, please refer to the following sources:
- Singh A, Lynch R, Solomon J, Weaver JD, May AR. 2023. Development of novel fluor mop materials for remediation of perfluoroalkyl substances (PFAS) from groundwater. J Hazard Mater 448:130853. doi:10.1016/j.jhazmat.2023.130853 PMID:36709737 PMCID:PMC1000247
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


