Superfund Research Program
Engineering Hydrogel Beads to Enhance Bioremediation of Groundwater Contaminant
Release Date: 05/01/2024
![]() subscribe/listen via iTunes, download(6.7MB), Transcript(52KB)
subscribe/listen via iTunes, download(6.7MB), Transcript(52KB)
Oregon State University scientists and engineers developed an approach to cleaning polluted groundwater that uses tiny beads containing chemical-eating bacteria. In this study, funded by the NIEHS Superfund Research Program (SRP), the team identified a formula to maximize bead durability and bioremediation, or the removal of contaminants using bacteria.
The chemical cis-1,2-dichloroethylene (cDCE), used to produce wax and solvents and to extract fat from animal products, is a prevalent groundwater contaminant associated with liver, kidney, and cardiovascular problems. Although some microorganisms can break down cDCE, conventional methods of stimulating microbial action may inadvertently disperse the chemical as airborne droplets.
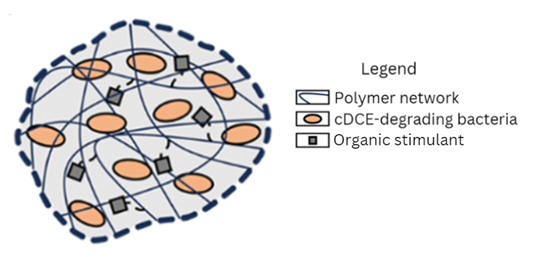
Previously, the team developed a treatment system that enmeshed cDCE-degrading bacteria and a growth-stimulating compound within beads of hydrogel — a substance made up of long chains of atoms, or polymers, that can absorb a large amount of water. The researchers packed the beads into columns and deployed them into contaminated water. However, the beads deteriorated quickly, which limited their use in bioremediation.
Creating and Testing Bead Variants
The researchers sought to improve their earlier design by developing and testing hydrogel beads made with different ratios of a synthetic polymer and alginate, a natural polymer produced by brown seaweed. Their goal was to create beads that could be packed tightly, retain elasticity over time, and promote efficient cDCE degradation.
To create the beads, the investigators mixed different amounts of the two polymers, as well as the cDCE-degrading bacteria and growth stimulant, into a chemical bath to link the polymers. Next, they incubated the beads in bottles containing a salt solution for 30 days, adding cDCE on the first and last day of incubation.
After incubation, the team analyzed bacterial activity by comparing how much oxygen the microbes had consumed to how much cDCE they broke down. Finally, they measured bead durability and elasticity by pressing individual beads between two metal plates every five days and measuring how much the beads deformed at 0.01 newtons of force.
Finding an Optimal Bead Formula
Bacteria in beads with higher levels of both polymers were generally more efficient at removing cDCE. Those beads were also more resistant to compression. For all formulas, elasticity decreased over the 30-day period, but beads with higher levels of both polymers retained more of their elasticity at the end of incubation.
Additionally, for all formulas, the rate of oxygen use decreased and the rate of cDCE degradation increased. In other words, the bacteria became more efficient over time, according to the authors.
Through statistical mathematical modeling, the researchers calculated the optimal hydrogel formula to maximize bead durability and elasticity and bacterial activity. Using this formula, they created new beads and tested their performance. Bead elasticity and bacterial activity generally aligned with their predictions, but the oxygen use rate was more variable than expected. The variation may stem from bacterial-hydrogel interactions that were unaccounted for in the models, according to the authors.
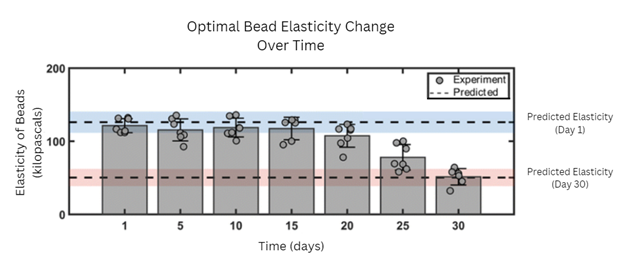
These optimal beads hold potential for long-term bioremediation given their durability and efficient microbial activity, the authors added. They recommended that future studies scale up production of these beads and test their performance in samples of contaminated groundwater.
For More Information Contact:
Lewis Semprini
Oregon State University
School of Chemical, Biological, and Environmental Engineering
316H Johnson Hall
Corvallis, Oregon 97331-3212
Phone: 541-737-6895
Email: lewis.semprini@oregonstate.edu
Willie Ernest Rochefort
Oregon State University
School of CBEE, 116 Johnson Hall
125 SW 26th St
Corvallis, Oregon 97331
Phone: 541-737-2408
Email: rochefow@oregonstate.edu
Michael R. Hyman
North Carolina State University
Department of Plant and MIcrobial Biologyering
203 Peele Hall
Raleigh, North Carolina 27695
Phone: 919-515-7814
Email: mrhyman@ncsu.edu
To learn more about this research, please refer to the following sources:
- Harris C, Gedde H, Davis A, Semprini L, Rochefort WE, Fogg K. 2024. The optimization of poly(vinyl)-alcohol-alginate beads with a slow-release compound for the aerobic cometabolism of chlorinated aliphatic hydrocarbons. RSC Sustain doi:10.1039/D3SU00409K
To receive monthly mailings of the Research Briefs, send your email address to srpinfo@niehs.nih.gov.


