Superfund Research Program
October 2023
By Lauren Sprouse
NIEHS Superfund Research Program (SRP)-funded researchers explored a new, cost-effective method of water treatment using biochar — a conductive, absorbent material — made from banana peels. This approach could inform large-scale, low-cost treatments in water systems, according to the authors at the Northeastern University Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) SRP Center.
Biochar is a type of charcoal formed by burning organic matter in an oxygen-free environment and is commonly used in the agricultural industry as a soil amendment. The properties that make biochar an effective soil additive — a porous structure, cost-effectiveness, and eco-friendly characteristics — also make it a promising option for water cleanup.
“Our center studies Superfund contamination in Puerto Rico, and considering the island’s tropical climate and thriving fruit production, we thought of exploring locally produced biomass as a way to create biochar,” said Akram Alshawabkeh, Ph.D., director of the PROTECT SRP Center and principal investigator on the studies. “The approach not only promotes sustainability but may also empower the local community by centralizing their production of water treatment materials.”
Creating a Cathode

In a recent study, PROTECT researchers developed and tested a biochar-based cathode — part of an electrochemical device that uses water to generate hydrogen peroxide and hydroxyl radicals, which are used in water treatment due to their ability to break down organic contaminants. Over time, hydrogen peroxide breaks down into water and oxygen, making it safe to use in drinking water treatment.
Traditional cathodes are created from metals, which are often expensive and run the risk of dissolving heavy metals into water. Carbon-based cathodes, such as those containing biochar, have been shown to produce hydrogen peroxide just as efficiently and with fewer risks.
To create their cathode, the PROTECT team heated banana peel waste and packed the resulting biochar material in stainless steel mesh, which distributes electric current through biochar, for use in their model water treatment system.
“What sets our banana peel-based biochar apart is its practicality and versatility,” Alshawabkeh said. “We can precisely control its surface chemistry by adjusting production conditions, like burning temperature and time, allowing us to tailor the biochar for targeted contaminants.”
Cleaning Up Contamination
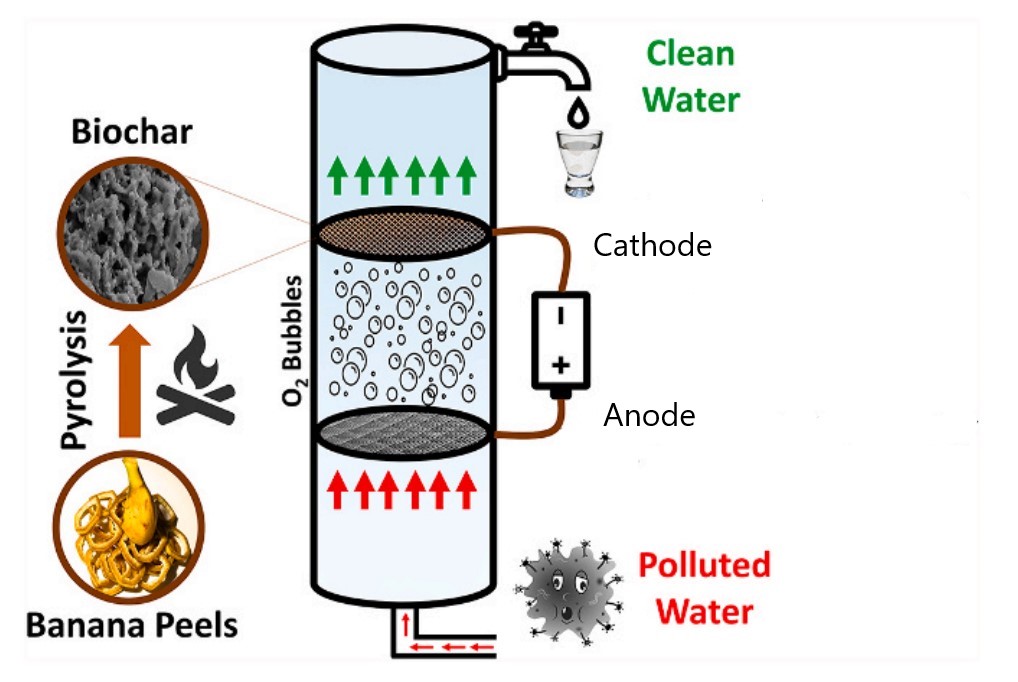
The researchers tested the efficiency of their cathode system using water with dissolved ibuprofen, simulating pharmaceutical contamination. Initial test results resulted in removal of just 40% of the ibuprofen.
The team then conducted additional experiments, such as adding persulfate — a chemical compound containing sulfur — to the system. Biochar can activate persulfate to produce sulfate radicals, which are highly reactive chemicals that can destroy organic contaminants.
With the addition of persulfate, they found 100% removal of ibuprofen.
“Our banana peel biochar proved to be just as effective as commercially available, coal-derived carbon sources, with the additional benefits of cost-effectiveness and sustainability,” Alshawabkeh said.
Changing Biochar Chemistry
Though the addition of persulfate significantly enhanced biochar’s cleanup abilities, adding persulfate to large-scale drinking water treatment systems is not feasible or desirable. The team looked into activating biochar, a way to change the biochar’s surface, which enhances its ability to produce hydrogen peroxide and subsequently degrade contaminants. They tested two different biochar activation methods — acidification and pre-anodization — and compared them to biochar that was not activated.
Acidification involved soaking the biochar in nitric acid to activate its chemical structure. Biochar pre-anodization, on the other hand, required changing the direction of electrical current to activate the biochar with using additional chemicals.
The researchers used blue and red dyes as model contaminants, which made it easier to assess changes in their concentrations by observing changes in color.
Compared to the acidified and non-activated biochars, the pre-anodized biochar showed a greater ability to conduct electricity, as well as significantly increased hydrogen peroxide production. The researchers also tested the efficacy of a polarity reversal approach to improve cathode stability and found that it is a cost-effective approach, and can be reused over multiple cycles while still removing a high percentage of dyes.
“These findings are applicable not only to water treatment, but also to renewable energy production, battery recycling, and more,” Alshawabkeh said. “Biochar bridges sustainability with real-world applications, addressing global challenges in energy and environment.”


